Why Do Some Galvanized Parts Look Shiny While Others Look Dull?
Hey there, rigging enthusiasts! We recently got a curious question from a customer who noticed that some of their galvanized parts had a shiny finish while others looked dull and was concerned about their effectiveness. Let's dive into the science of galvanizing to clear things up.
The Galvanizing Process
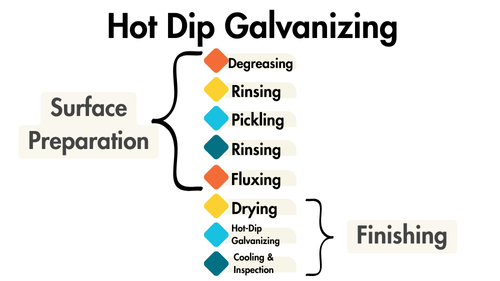
Hot dip galvanizing is a widely used method for protecting steel parts from corrosion. Here's a detailed look at how the process works:
-
Surface Preparation: Before galvanizing, the steel parts
must be thoroughly cleaned. This involves several steps:
- Degreasing: Removing oil, grease, and dirt using an alkaline or acidic solution.
- Pickling: Immersing the steel in a bath of hydrochloric or sulfuric acid to remove rust and mill scale.
- Fluxing: Coating the cleaned steel with a layer of flux (typically zinc ammonium chloride) to prevent oxidation before dipping in molten zinc.
-
Galvanizing: The cleaned and fluxed steel parts are then dipped into a bath of molten zinc, which is typically maintained at a temperature of around 450°C (840°F). The immersion time depends on the size and thickness of the parts but usually lasts a few minutes. During this stage, the following happens:
- Zinc-Iron Reaction: The zinc reacts with the iron in the steel to form a series of zinc-iron alloy layers. This metallurgical bond provides excellent corrosion resistance.
- Surface Finish: As the parts are withdrawn from the zinc bath, the zinc solidifies and forms a coating. The appearance of this coating can vary from shiny to dull.
-
Post-Galvanizing Treatment: After galvanizing, the parts may undergo additional treatments:
- Quenching: Sometimes the parts are quenched in a passivation solution to cool them quickly and prevent the formation of excessive zinc-iron alloy layers.
- Inspection: The galvanized parts are inspected for coating thickness, uniformity, and adherence to quality standards.
Behind the Sheens
Several factors contribute to the varying appearances of galvanized parts:
-
Elemental Differences: The chemical composition of the steel, especially the silicon content, affects the finish. Silicon acts as a catalyst in the zinc-iron reaction. Higher silicon levels lead to a faster reaction and a thicker, duller coating.
-
Cooling Rates: The rate at which the parts cool after being dipped in zinc can influence their sheen. Thinner sections cool faster, often retaining a shiny finish. Thicker sections cool more slowly, allowing for the formation of a duller, matte surface.
-
Batch Cooling: When parts are cooled in batches, their position within the batch can impact their surface finish. Parts that are closer to others may cool more slowly and develop a duller appearance, while those more exposed cool faster and appear shinier.
What Does This Mean for Corrosion Protection?
The difference in appearance does not affect the corrosion protection offered by the galvanized coating. Both shiny and dull finishes provide the same level of protection because:
- Thickness and Durability: Dull coatings are often thicker, providing superior resistance to abrasion and corrosion.
- Uniform Weathering: Over time, all galvanized coatings will weather to a uniform dull grey when exposed to the elements. This natural aging process does not affect the protective qualities of the zinc coating.
So, if your galvanized parts look a bit different, don't worry! They're all up to industry standards and ready to perform.